ProtTech offer the service for the complete de novo sequencing of mAb and other proteins. The service is based on the proprietary protein sequencing technology that we developed over many years.
We guarantee a result with a 100% sequencing coverage of the entire mAb molecules, a better than 99.5% accuracy and distinct assignment of Leu/Ile residues.
Procedure:
The complete mAb de novo sequencing is often a very complex process, involving more than ten different proteolytic digestions, several steps of chemical derivatizations, NanoLC-ESI-MS/MS data acquisition, bioinformatics analysis with more than a dozen proprietary softwares, the manual spectra assignment, etc. In general the process contains the following steps:
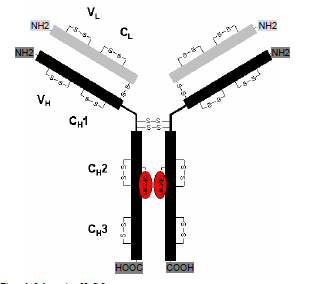
1). Proteolytic digestion and chemical derivatization.
2). LCMS data acquisition.
3). Database search and peptide de novo sequencing.
4). Draft sequencing assembling.
5). Sequence gap filling.
6. Sequence error screening.
7). I/L determination.
8). Sequence validation.
Our mAb de novo sequencing technology can also apply to other proteins. However, changes in the process is often needed based on the nature of the protein.
Sample requirement:
Since more than ten different proteolytic reactions are carried out in the sequencing process, we prefer to have > 1mg mAb protein for each project, although ~500ug mAb sample is acceptable. A better than 90% purity is required.
The main concern about the quality of a sample is immunoglobin contamination since there is no easy way to separate contaminating antibodies from the mAb of interest, and there is not easy to determine if a peptide from contaminating peptides or from the mAb of interest. The major source of such a contamination is the serum in cell culture media.
Turn-Around Time:
It often takes five week to sequence one mAb sample.


